Home Food Safety Food Safety Services for Importers & Exporters
Food Safety Services for Importers & Exporters
The United States imports nearly $12 billion worth of seafood every year. The U.S. Food and Drug Administration (FDA) is becoming more stringent in its enforcement of existing regulations, which translates to detention of seafood products due to the presence of pathogens, improper labeling, filth and decomposition.
If denied entry to the United States, these products either have to be reconditioned, relabeled or discarded depending on the condition of the product and the violation. Here are several of the most common reasons that your food might be prevented from entering the United States.
- Filth - Shrimp imported into the United States is often detained for being described as "filthy." According to the FDA, filthy shrimp "appear to consist appear to consist in whole or in part of a filthy, putrid or decomposed substance or that appear to be otherwise unfit for food."
- Microbial contamination - The FDA rejects about 30-40% of its imported shrimp due to microbial contamination. Most often, the offending organism is salmonella, a gram negative bacteria that can cause a variety of infections when consumed. When this type of organism is identified, it is most usually due to problems associated with poor plant hygiene among workers or problems associated with the plant's HACCP program.
- Labeling - Many countries have problems meeting the stringent labeling requirements of the United States. Labeling problems are not just limited to non-English speaking countries. This violation is probably the most common in all products imported into the United States. The FDA defines a labeling violation as "the article appears in violation of FPLA because of its placement, form and/or contents statement."
- HACCP violations - All processors exporting seafood to the United States must not only ensure that their product is free of contaminants, but they also must have a HACCP system in place that eliminates the potential for microbiological, physical and chemical contamination.
How Can EHA Help You?
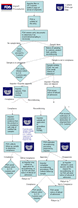
- Meeting with the FDA on behalf of the exporter/consignee to understand how the product can be released into commerce.
- Working with nationally certified laboratories to present records of evidence and samples to indicate the product is in compliance or at the least within the prescribed guidelines for the level of contaminants.
- Proposing and overseing a protocol, for additional testing and/or reconditioning of products, for acceptance by the FDA.
- Helping the importer/consignee with re-labeling and bringing the product into compliance.
- HACCP-based inspection of your manufacturing/processing facility and training employees on HACCP principles.
EHA offers unique and targeted expertise on matters including FDA compliance and quality assurance. Our services involve auditing, due diligence, loss prevention, crisis management, product reconditioning and relabeling, training, and litigation support. Our team offers an ""insider's" perspective, having scientific/legal/regulatory backgrounds with FDA-regulated products. We strive to help prevent problems before they occur, as well as to resolve promptly any that may already exist.
Our corporate offices are based in the Baltimore-Washington area, but our consulting practice is global. We have extensive experience with domestic and foreign entities, as well as foreign language capabilities. From our headquarters, we are able to call upon vast resources from academic centers and other public and private facilities. Our team includes epidemiologists, registered pharmacists, attorneys, former health department officials, and university professors. We offer extensive experience and corresponding credentials as experts in our fields.
Our consultations encompass assistance with matters involving FDA, USDA, DEA, and state agencies, reimbursement, cost-containment, advertising and promotion issues, audits, risk reduction and quality assessment in the practice environment.
Contact EHA Consulting Group today for more information about how we can assist your company.
Diseases, Infections, or Illnesses Acquired
We offer services for
- Retail Food Safety
- Restaurant Food Safety
- Manufacturers
- Cruise Ship Food Safety
- Food Trucks
- Importers & Exporters
- Drugs & Cosmetics
- Fruits & Vegetables
- Melons & Cantaloupes
- Produce
- Warehouses
- Food Packaging & Packaging Materials